Water treatment in today's modern world requires high technology, high-performance separation products to achieve increasingly stringent treatment water quality or to provide the lowest cost of water production. However, more than one separation technology is often applied to achieve the required quality.
The main reverse osmosis membrane structures are based on thin film composite membranes (see Figure 1). The barrier (or rejection) layer is a 0.1 μm thick polyamide layer supported by a polysulfone substructure. The polyamide layer is formed by a polymerization process. Although thin film laminates based on this process have been used for more than 30 years, the latest technology now offers the possibility to control the polymerization process more precisely. Therefore, a focus of our membrane development is to increase the degree of polymerization of the polyamide layer. A higher degree of polymerization improves the mechanical and chemical stability of the thin barrier layer, thus providing greater durability. In addition, the negative charge on the membrane surface is reduced, resulting in lower cation adsorption (fouling) on the membrane surface. Due to their chemical nature, polyamide membranes typically have a negatively charged surface and often result in cationic fouling that is extremely difficult to remove. A typical example of cationic contamination is iron contamination. Ferric chloride (FeCl3) is a very common flocculation chemical used in pretreatment systems. If the dose is too high, even for a short period of time, cationic contamination can irreversibly contaminate the RO membrane surface. In addition to a well-adjusted ferric chloride dosing system, a lower negative surface charge is the best option to reduce the potential for fouling in this event. The membrane separation composite layer is the most important part of the RO separation process. Prior to use, this critical component is assembled into a device called an RO element. The winding process for spiral wound RO elements involves many steps, all of which need to be carefully controlled. The LANXESS manufacturing process involves state-of-the-art robotic equipment to carefully prepare the RO element according to strict mechanical specifications. Much of this development is performed with the assistance of external agencies that apply modern computer-aided design capabilities to confirm mechanical strength and optimize the hydrodynamic design (see Figure 2). This critical development process is necessary to chemically assemble improved membranes into modern RO elements.
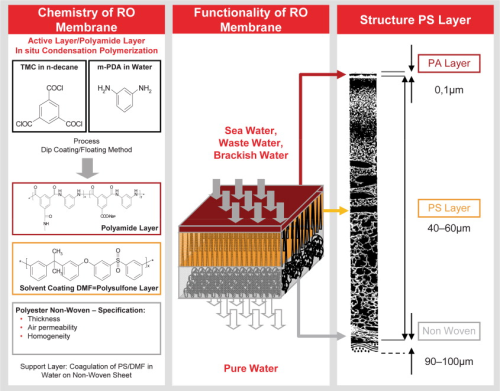
Figure 1: Structure of a thin film composite membrane.
Figure 2: Pressure testing of the anti-stretch device (ATD) and permeate tube.
The new reverse osmosis membrane elements were placed in an existing RO unit that treats 40m 3 /hr of Rhine water after an ultrafiltration system. A total of six elements were installed in pressure vessels. the RO system also contained several pressure vessels containing RO elements from another supplier. The components were installed about a year ago and the pressure vessels operate in parallel. The entire RO system operates in a two-stage system with a 6:3 array using a six-element pressure vessel. It can be demonstrated that we provide flux values of the same order of magnitude when compared to elements installed by other vendors. TOC and silica retention were measured periodically during field testing, while TOC retention was measured at approximately 95-96% and total silica retention was measured at approximately 99.3%. In summary, the new RO membrane elements were demonstrated to operate in a similar manner under the same operating conditions.
| Process | Demineralisation | Desalination |
| Limits | Conductivity < 2 μS/cm TOC < 500 ppb SiO2 < 50 ppb | Conductivity < 0.055 μS/cm TOC < 100 ppb SiO2 < 10 ppb |
| Technology | Ion Exchange Reverse Osmosis Electrodialysis | Ion Exchange (mixed bed) Electrodeionisation (EDI) |
The reason UltraClean took the step to add RO membrane technology to its separation portfolio is that reverse osmosis is a complementary technology to ion exchange (IX) resins. In general, reverse osmosis can effectively desalinate water with high salinity, but ion exchange can selectively remove certain ions from water. Table 1 shows the separation technologies that can be used, depending on the required permeate quality. In modern separation applications, the combined RO and IX process is used not only for applications such as boiler feedwater desalination, but also for other process applications such as boron removal from seawater or treatment of extracted water from unconventional natural gas resources. These applications have recently been widely discussed due to increasing public concern about water quality and environmental factors. Natural gas production from unconventional sources is growing rapidly worldwide. The water that comes with the gas is not only difficult to treat, but often must meet strict regulatory standards before it can be released into the environment. These regulations present a challenge to water treatment system designers. Although highly variable, typical waters can range from 2,500-10,000 mg/l TDS, 1,000-3,000 ppm total alkalinity (in CaCO), and pH on the order of 8-9. Because most inland areas are sensitive to salt releases, the water treatment process of concentrates require specialized treatment. Minimizing the volume of waste concentrate is therefore critical to the success of the application. An RO process with high recovery rates and low chemical use is one solution. To achieve this, the use of IX as a pre-treatment softening and inter-stage treatment in a three-stage single-pass RO unit is an option.
In order to achieve high recovery, hardness needs to be softened to the ppb level. In order to achieve this reliably, especially for high salinity water, the selective IX process is used. One example is the weakly acidic cationic resin type, which is typically used prior to reverse osmosis treatment of brackish water. Another example is the chelate resin, which is able to effectively soften to the ppb level even with saturated brine solutions. This type is often used to soften concentrates from RO plants prior to further RO treatment. When strontium and barium removal is important, iminodiacetic acid chelate resins are usually chosen; otherwise, aminophosphonic acid resins are used in preference. In most applications, chemical treatment with acids or scale inhibitors is used as pretreatment for the RO process. IX softening processes have the advantage that salt discharge is difficult, as in the example described, or if the salt solubility product (Ksp) is well above the saturation limit and therefore scale inhibitors cannot be used in the application (see Table 2).
| Scale forming compound | Conservative saturation level |
| CaSO4 | 230% |
| BaSO4 | 6,000% |
| SrSO4 | 800% |
| SiO2 | 150% (or 200ppm) |
| CaCO3 | LSI > 1.8, SDSI >1.0 |
Contrary to the example of extracted water treatment described, IX is used as a post-treatment for boron removal. The boron removal using RO alone was performed at pH 9. At this pH, the boron fraction is negatively charged and can be removed by up to 90% for seawater RO and up to 75% for brackish water RO elements. To get the boron content in the permeate below 0.5 mg/l, an additional RO treatment of the first permeate (via a two-pass or partial two-pass system) is required. In this case, pH adjustment is performed before the second pass. An alternative to this process is to use IX for post-treatment. Although only a few plants have installed this technology, this process has some clear advantages if the customer requires a low boron concentration (0.3 mg/L). In pilot tests at desalination plants, boron levels can be reduced from 0. 7 mg/L (post-RO) to 0.2 mg/L (working capacity 2.6 g/L). Since IX is a highly selective separation process that removes mainly boron, the capacity of the resin is not depleted by other ions. A similar process can be used to selectively remove other key compounds, such as arsenic or heavy metals, after the RO process. Most engineers use the product manufacturer's custom software to design ion exchange or reverse osmosis systems. When economically validating RO and IX processes, the cost of the discharge concentrate is often important. However, the salt concentration of the feed is usually the most important. While the specific cost of deionized water using IX depends on the salt concentration of the feed water, the specific cost of an RO plant is constant for a wide range of salt concentrations. On the other hand, the specific cost of RO treated water starts at a higher level, so the break-even point (crossover point) indicates to the designer that the salinity values for IX and RO have the same cost. In addition to economic validation, other reasons may arise as to why RO or IX was chosen for the process. In general, if ease of handling is a key selection issue, the RO process is preferred, while if high selectivity is beneficial, IX is preferred.
UltraClean believes that IX and RO technologies will continue to grow strongly in the near future. Desalination is growing rapidly, with a projected growth rate of 12%; brackish water is growing at a slightly lower rate, and RO membrane processes clearly have a bright future. In addition, IX also has a bright future as water treatment processes require higher efficiency and selectivity. Modern water treatment technology requires a combination of technologies. For example, the integration of different membrane processes, such as ultrafiltration and RO membrane separation, or a combination of several technologies such as RO and IX, or RO and EDI.